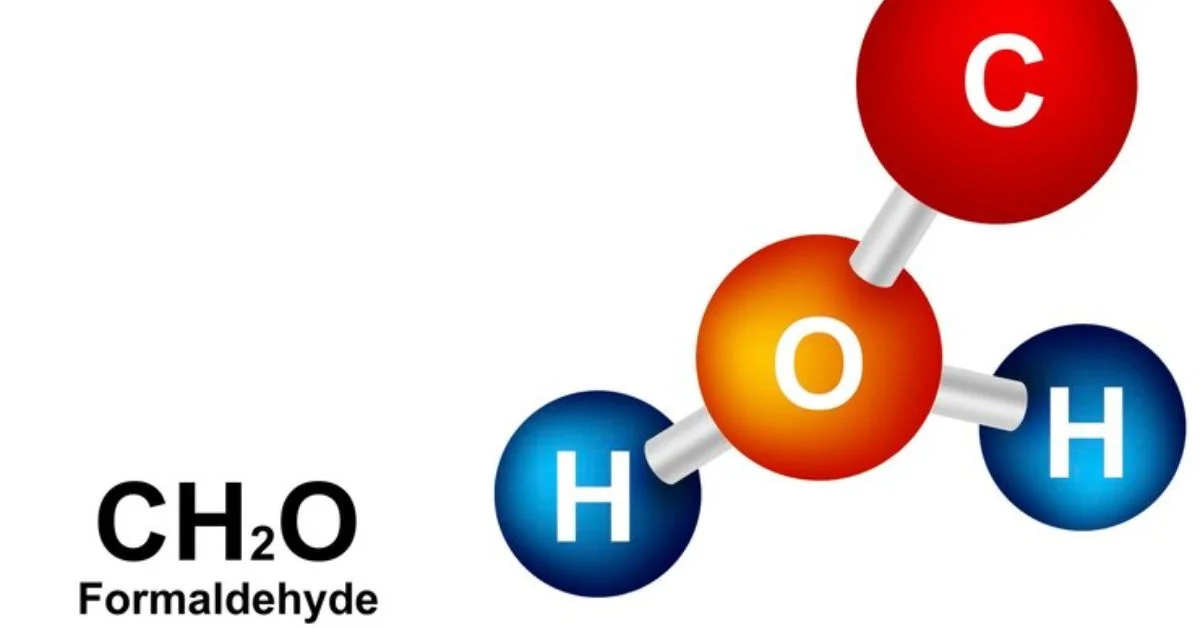
While the chemical world is vast and ever-changing, some molecule combinations—like HCOOH CH2O H2O—have captured the attention of scientists for their peculiar properties and potential uses. The importance, reaction process, and industrial relevance of formic acid, formaldehyde, and water are explored in this article.
If you’re a student, chemist, or just someone interested in learning more about the process, this article will help you understand it by breaking it down into easy steps while still being technically accurate.
Overview of the Reactants
Formic Acid (HCOOH)
Formic acid is the simplest carboxylic acid and is naturally found in ant venom. It’s a colorless liquid with a strong, pungent smell.
- Molecular formula: HCOOH
- Molar mass: 46.03 g/mol
- Usage: Preservative, antibacterial agent, and industrial reagent
Formaldehyde (CH2O)
Formaldehyde is a gas at room temperature but usually handled in aqueous form (formalin).
- Molecular formula: CH2O
- Molar mass: 30.03 g/mol
- Usage: Disinfectant, resin production, and chemical intermediate
Water (H2O)
Known as the universal solvent, water plays a crucial role in facilitating chemical reactions.
- Molecular formula: H2O
- Molar mass: 18.02 g/mol
- Usage: Solvent, diluent, and reaction medium
What Happens in the Reaction HCOOH CH2O H2O?
Condensation is the result of mixing formic acid (HCOOH) with formaldehyde (CH2O) in water. Methylene glycol derivatives, and in some regulated situations, hydroxymethyl derivatives, are major products of this process.
This process involves more than just neutralization; it involves the change of functional groups to produce new molecules.
Mechanism and Chemical Equation
Formic acid has a nucleophilic addition reaction with formaldehyde in aqueous media. Oxygen from molecules of acid or water can damage the carbonyl group of formaldehyde.
General Equation:
HCOOH CH2O H2O → HOCH2COOH (Hydroxymethylformic acid)
Steps in the Mechanism:
- Protonation of formaldehyde by HCOOH
- Nucleophilic attack on the carbonyl carbon
- Formation of a gem-diol intermediate
- Rearrangement or stabilization to hydroxymethylformic acid
Applications of the Reaction
- Textile Industry: Used in resin finishing agents to improve wrinkle resistance.
- Adhesive Manufacturing: Components in urea-formaldehyde adhesives.
- Pharmaceutical Intermediates: Synthesis of drug precursors.
- Agricultural Chemicals: Forms part of herbicide and pesticide formulations.
Conditions Influencing the Reaction
The outcome of the hcooch ch2 h2o reaction can be heavily influenced by:
- pH Levels: Acidic conditions favor nucleophilic attack.
- Temperature: Higher temperatures increase reaction speed.
- Solvent Purity: Impurities can alter the yield.
- Concentration: Optimal molar ratios are essential.
Environmental and Industrial Importance
- Biodegradability: Products of this reaction, especially when not polymerized, are biodegradable.
- Industrial Use: Found in the synthesis of environmentally friendly adhesives.
- Green Chemistry: Water as a solvent makes this a more sustainable option.
Related Reactions and Extensions
- Cannizzaro Reaction: Involving aldehydes in basic conditions, which contrasts the acidic medium here.
- Mannich Reaction: Another example of formaldehyde participating in complex molecular transformations.
- Polyoxymethylene Formation: Formaldehyde polymerizes under certain conditions.
Safety Considerations
Handling Guidelines:
- Formic Acid: Corrosive. Use gloves and eye protection.
- Formaldehyde: Carcinogenic. Use in well-ventilated areas.
- Water: Generally safe but avoid contamination.
Storage:
- Store reactants separately in tightly sealed containers.
- Label clearly and keep away from children.
Conclusion
More than just a simple chemical equation, the HCOOH CH2O H2O reaction has deeper implications. It embodies practical significance in the fields of green chemistry, medicine, and manufacturing. By learning about this reaction, you may enhance your understanding of organic processes and get a better appreciation for its practical uses in many sectors.
Researchers, scientists, and teachers may all benefit from this reaction as a fantastic example of a process that combines theory and practice.
FAQs
Q1: What is the product of HCOOH and CH2O in water?
The reaction forms hydroxymethylformic acid or related hydroxyl derivatives.
Q2: Is this reaction reversible?
Under certain conditions, yes. Heating or pH changes may shift equilibrium.
Q3: What industries use this reaction?
Textile, pharmaceutical, adhesive, and agricultural industries.
Q4: Are the reactants dangerous?
Yes. Formic acid is corrosive and formaldehyde is carcinogenic. Handle with care.
Q5: Can this be performed in a school lab?
With proper safety protocols, yes, though formaldehyde use may be restricted in some educational environments.
For more information, click here.